How Do I Qualify to Read Breast Mri
- Review
- Open Admission
- Published:
How I report breast magnetic resonance imaging studies for breast cancer staging and screening
Cancer Imaging volume 16, Article number:17 (2016) Cite this article
Abstruse
Magnetic resonance imaging (MRI) of the breast is the virtually sensitive imaging technique for the diagnosis and local staging of primary breast cancer and nevertheless, despite the fact that it has been in utilize for 20 years, there is fiddling show that its widespread uncritical adoption has had a positive touch on patient-related outcomes.
This has been attributed previously to the low specificity that might exist expected with such a sensitive modality, but with modern techniques and protocols, the specificity and positive predictive value for malignancy can exceed that of chest ultrasound and mammography. A more likely explanation is that historically, clinicians take acted on MRI findings and contradistinct surgical plans without prior histological confirmation. Furthermore, modern adjuvant therapy for breast cancer has improved then much that it has become a very alpine order to show a an comeback in outcomes such as local recurrence rates.
In society to obtain clinically useful information, it is necessary to understand the strengths and weaknesses of the technique and the physiological processes reflected in breast MRI. An advisable indication for the browse, proper patient preparation and good scan technique, with rigorous quality assurance, are all essential prerequisites for a diagnostically relevant study.
The apply of recognised descriptors from a standardised lexicon is helpful, since assessment tin can then dictate subsequent recommendations for direction, as in the American College of Radiology BI-RADS (Breast Imaging Reporting and Information System) lexicon (Morris et al., ACR BI-RADS® Atlas, Breast Imaging Reporting and Information System, 2013). Information technology also enables audit of the service. Nonetheless, perhaps the most critical gene in the generation of a meaningful report is for the reporting radiologist to have a thorough understanding of the clinical question and of the findings that will influence management. This has never been more important than now, when nosotros are in the throes of a remarkable image shift in the treatment of both early on stage and locally avant-garde breast cancer.
Background
The sensitivity of mammography for breast cancer detection in women over 50 years is well over lxxx % [one] and in the symptomatic population, when combined with chest ultrasound (US), this figure increases to effectually ninety %. It might so exist asked why another imaging modality such as breast MRI is required at all. However, it is well recognised that the sensitivity of mammography is essentially lower (around 50 %) in the mammographically dense breast, even with state of the art full field digital mammography (FFDM) [2]. Furthermore there is limited inherent contrast in mammography; many lesions are indeterminate, requiring further evaluation and biopsy; there are recognised observer limitations and information technology requires radiation (albeit low dose) and breast compression, which most women observe very uncomfortable. Though many of these limitations are negated past high quality US, this too is operator dependent, often misses microcalcifications (the mammographic hallmark of ductal carcinoma in situ, DCIS), and also suffers from depression specificity especially in the screening setting [three, 4].
On the other manus, dynamic dissimilarity-enhanced breast MRI (DCE-MRI), the 'breadstuff-and-butter' MRI technique for breast cancer detection, has a sensitivity for invasive cancers greater than 95 % in most series [5] and is the most authentic imaging technique for neoplasm size assessment in virtually circumstances [6, 7]. It can detect additional ipsilateral foci of disease in the breast known to harbour a cancer in upwards to as many as 25 % of cases [8], and detects synchronous contralateral occult disease with a median frequency of iv % [9]. There are some reports suggesting that information technology is amend than mammography for the detection of DCIS, peculiarly more aggressive biologically relevant high grade DCIS [x]. Chiefly, numerous studies have shown that DCE-MRI of the chest is a far more than sensitive screening modality than FFDM or United states of america in the detection of clinically occult chest cancer in women at greatly increased lifetime hazard, especially those with BRCA mutations [xi–14], with near studies showing a doubling of the cancer detection rate with breast MRI and fiddling boosted benefit from mammography.
So why is breast MRI not used more frequently in routine practice? In countries that have resource-limited healthcare systems, such equally the United kingdom of great britain and northern ireland National Wellness Service (NHS), timely admission to MRI is a major issue, but even in resources-rich countries such equally the USA, many insurance providers are refusing to reimburse chest MRI studies except in certain well divers scenarios. Most centres take seen an exponential increase in need for breast MRI, yet to engagement, despite numerous studies demonstrating the superiority of chest MRI over conventional imaging in local staging, this has not translated into beneficial patient related outcomes. Specifically, the bear witness suggests that employ of preoperative breast MRI in patients with breast cancer results in increased mastectomy rates or larger wide local excisions [15] with, at the same time, no reduction in the incidence of positive surgical margins (necessitating re-excision) [16, 17] nor, ultimately, in ipsilateral in-breast local recurrence [18]. Similarly, though there is expert evidence of stage shift as a result of the use of breast MRI for screening of high-adventure women [xix], it remains to exist seen whether the increased cancer detection charge per unit with breast MRI in high take a chance women translates into improvements in breast cancer-specific bloodshed [20], at least in the BRCA ane population.
What this seeming paradox tells united states of america is that breast MRI should exist used judiciously; this is my own accept on how to make information technology as useful equally possible. There should be a very good indication for carrying out breast MRI and though space precludes a detailed exploration of the indications here, the situations in which I would either advocate or consider breast MRI for local staging are listed in Table 1. This is by no means an exhaustive list and generally, difficult and fast rules are unhelpful. It is my business firm view that decisions most whether or non a chest MRI is appropriate should exist taken in the MDT meeting subsequently thorough discussion. For example, have a patient with a pre-operative diagnosis of invasive lobular carcinoma (ILC). Many centres would routinely obtain a breast MR in any such patient and there is limited testify from single heart studies that information technology may reduce the incidence of positive surgical margins without an increase in mastectomy rates [21]. Nevertheless, it is unnecessary if conventional imaging has shown clearly that the disease is multicentric and that breast conservation is not an selection. Similarly, the bear witness for a higher rate of synchronous contralateral malignancy with ILC has been overstated [22] and screening of the contralateral chest is not more often than not indicated. On the other hand, there may be genuine uncertainty well-nigh the local extent of illness, notwithstanding if the patient's comorbidities prevent surgical resection, there is no point in obtaining a chest MRI. Therefore, choose your indication and your patient carefully!
Finally, it is incumbent on usa to exist enlightened of shifting handling paradigms; with increasingly sophisticated oncoplastic surgical techniques, MR demonstration of multifocal disease or even segmental DCIS over equally much every bit 6 or 7 cm need not foreclose breast conservation.
Maximising the chances of obtaining a diagnostically useful study
In one case the conclusion to obtain a breast MRI has been fabricated, it is of import to stack the odds in your favour. I volition not carry out a breast MR scan unless I have access to all relevant prior imaging, whether it exist conventional mammography or MRI, and all clinical details including timing of previous biopsies or interventions, surgery or radiotherapy, and any histology results. All also often clinical details country 'right breast cancer? extent' – this is inadequate!
If the scan is elective, for example in the loftier run a risk screening setting, the scan should be scheduled for around twenty-four hours 10 of the menstrual bike and if the patient is on HRT it may be necessary to discontinue this for half-dozen weeks to minimise misreckoning groundwork chest parenchymal enhancement (BPE). In my experience this suggestion is ofttimes not well received, but on the other hand, this patient cohort is very highly motivated. In the patient with known breast cancer, such scheduling is non possible and note should be made of the date of the terminal menstrual catamenia and hormone replacement therapy (HRT) usage.
Patient preparation is extremely important. Information technology is impossible to overemphasise the function of sympathetic MR technicians who can talk the patient through the process and – critically - who are non afraid to manipulate the breast in order to optimise patient positioning within the breast coil. Cod liver oil capsules taped to the skin can be useful to marker scars or the site of the clinical abnormality. The patient must lie prone without moving for a minimum of 25 min and comfort is essential. In my unit, the patient data sheet warns patients to avert a large meal prior to the browse equally this tin can brand lying prone for the process very uncomfortable. The more fourth dimension spent ensuring the breasts lie centrally within the scroll, with no skin folds, the meliorate. If the MR technicians are not trained mammographers, I recommend a trip to the breast unit to proceeds some feel of mammography and patient positioning. Often the first scan acquired is degraded by motion artefact, so information technology is a good idea for this scan non to be the get-go of the dynamic series as it may exist necessary to repeat information technology.
Scan protocol
A detailed consideration of scan sequences and technical developments in sequences is outwith the scope of this article, but there are a few germane points. Though breast MRI scans can be considered to be more or less 'out of the box' at 1.5T, this is non truthful of scans at higher field strengths, when prior sequence optimisation on phantoms and good for you volunteers is essential, especially for sequences involving fatty suppression (specially improvidence weighted imaging), which can exist very problematical around the chest because of susceptibility effects induced by air/soft tissue interfaces.
Unless there is adept reason to believe that the patient will only tolerate one sequence - which should exist the dynamic T1 weighted gradient echo acquisition - I commence with a high resolution axial T2 weighted TSE sequence without fat suppression (voxel size 0.9 × 0.ix × 2 mm). This is very valuable for evaluation of the morphology of masses and identification of oedema, cysts and blood products, considered in conjunction with a non fat suppressed T1 weighted 3D slope echo sequence (oftentimes very useful for identification of marker clips). I follow this with diffusion-weighted imaging (DWI). In patients who are chest feeding or who take other contraindications to intravenous gadolinium-based dissimilarity, the written report tin stop at this signal and a surprising amount of information may exist obtained, especially in young women with prominent fibroglandular parenchyma [23]. Even so, the unmarried most important sequence remains the semi-dynamic T1 weighted gradient echo sequence, which can be 2d or 3D, with or without fatty suppression. I favour an axial 3D sequence with fat suppression, voxel size 0.nine × 0.nine × i.2 mm and acquisition time 45 southward. True pharmacokinetic analysis is not possible with this sequence, only it is not necessary outside the research arena. Nonetheless, it is important to appreciate the effect of browse duration on enhancement patterns; if browse time is prolonged, rapid enhancement of a mass and washout can be missed and with a slow injection rate, peak enhancement tin exist dampened (Fig. 1). Conversely, information on kinetics should non be acquired at the expense of spatial resolution. For this reason I scan out to around 6 min for the dynamic series and and then obtain a high resolution T1 weighted 3D slope echo sequence with water excitation and isotropic resolution, voxel size 0.7 mm3, caused in 4 minutes 30 s. This is an excellent sequence for morphology (for example, showing non-enhancing internal septations in fibroadenomata) and for those relatively rare malignancies such as low grade classical ILC, that may enhance relatively picayune and late.
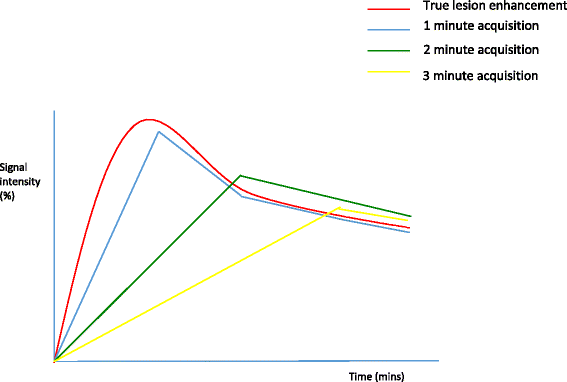
The effect of acquisition time on enhancement curves. A theoretical graphical depiction of the effect of dynamic acquisition time on credible contrast enhancement kinetics; injection at time 0. Bespeak intensity (%) vs. time (minutes)
How I read chest MRI studies
In my institution, the medical MR physicist and I look at the scans together on the modality workstation and generate time-intensity curves from any regions of interest on the subtracted dynamic series. These are after sent to PACS for reporting. If your institution has post-processing software this is very helpful, but I recommend that you ascertain from the articles and application specialists exactly what manipulations accept been carried out on the raw data; non all softwares are equal and there is a dearth of literature on the reproducibility of results (for example, functional tumour volumes) between vendors. This may non matter for a one-off diagnostic report, but information technology is extremely important if, for example, i is monitoring response to neoadjuvant chemotherapy.
My PACS hanging protocol is shown in Fig. 2. I like to see the T2 weighted sequences, the DWI and ADC map and the axial maximum intensity projections (MIPs) across the superlative row. If it is the showtime MRI written report, I will load the kickoff and second post-dissimilarity subtracted series, the delayed loftier-resolution studies and the post-processing paradigm captures along the bottom row. If it is a follow-upwardly study I will display the same sequences in height and bottom rows with the older examination beneath.
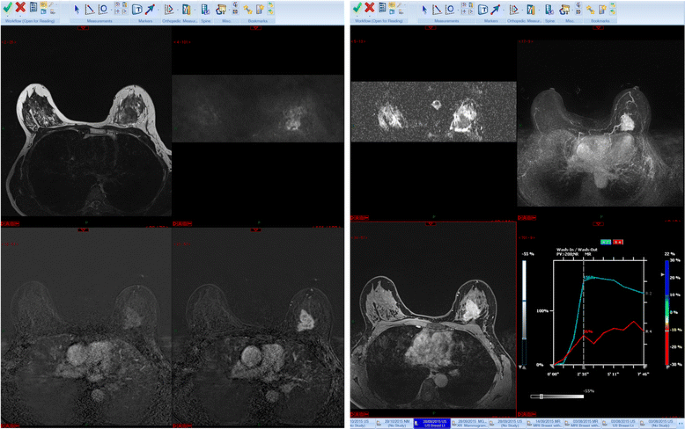
Hanging protocol. From left to right, T2 weighted, diffusion weighted series and corresponding ADC map, MIP images (top row). On the bottom row, from left to right, get-go and second subtracted series, loftier resolution post dissimilarity serial and post-processing
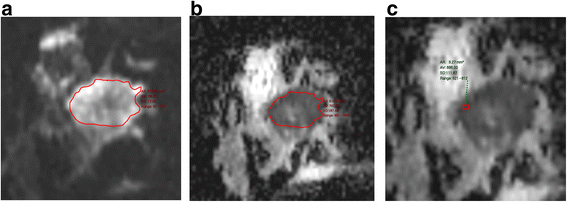
I start past having a quick await at the MIP series to ascertain a) whether in that location is any significant enhancement in the breasts and b) how much movement there has been during the acquisition. Secondly, I take a look at the first and second raw and subtracted series, remembering that if there has been much motility, the subtracted series tin exist totally misleading. Artefactual enhancement tin be recognised readily by the presence of alternate vivid white and black bands and can make sizing of a lesion hard peculiarly if there is suspected DCIS (Fig. 3a). Here reference to the raw data and the delayed loftier resolution sequence can be very helpful (Fig. 3b). However it may be necessary to state in the final written report that confidence in authentic sizing is limited. On the other hand, significant enhancement tin be obscured and evaluation of the morphology of a mass is challenging. Postprocessing softwares generally have an algorithm for motion correction, but there are limits to what can be achieved with this, peculiarly if motion is along the z axis.
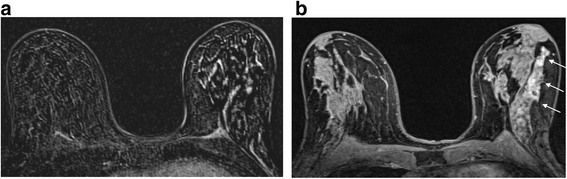
The event of motion on subtracted images. a Axial post-contrast subtracted prototype showing severe misregistration secondary to motion in the left chest. It is not possible to identify nor estimate the extent of the known high class DCIS in this patient. b Centric high resolution fat suppressed T1 weighted image post contrast. The non-mass segmental enhancement in the left breast is identifiable (arrows). At pathology there was twoscore mm of high grade DCIS
As well as looking for the presence of meaning enhancement I assess the degree of background parenchymal enhancement (BPE) around 2 min post injection of dissimilarity. This is alike to assessing the amount of fibroglandular parenchyma on a mammogram and has the aforementioned purpose; it should point the level of certainty about whether or not a significant lesion is present. Just as a cancer can exist obscured in the dense chest on mammography, so the presence of florid BPE tin render chest MRI interpretation difficult (Boosted file 1: Figure S4a and b). However, contrary to the situation with mammography, there appears to be no drop in the sensitivity with severe BPE, despite a college charge per unit of examinations chosen abnormal [24, 25]. In the latest BI-RADS dictionary, BPE is graded a to d for none/minimal through to severe; the meaningless attempt to assign a percentage figure that was present in the previous edition has, quite rightly in my view, been dropped. Care should exist taken to ensure that windowing is advisable; you should be able to 'see in' to the breast without making window widths so bully that enhancement cannot be appreciated. Note that BPE can be asymmetrical, equally shown in Additional file 1: Figure S4c in a patient who received unilateral whole breast adjuvant radiotherapy.
If abnormal enhancement is nowadays, I adjacent look at the T2W series for a morphological correlate. This yields useful information not only nearly the possible nature of a mass, but also, in the example of known cancers, the probable imaging phenotype. For case, Fig. 2 demonstrates the typical MR appearances of a grade 3, hormone receptor negative cancer. The T2W scan can besides enable ane to dismiss small foci of enhancement and tin be very useful to ostend, for case, that an ovoid focus of enhancement with washout is in fact an intramammary lymph node. Linking the various series together makes this process straightforward.
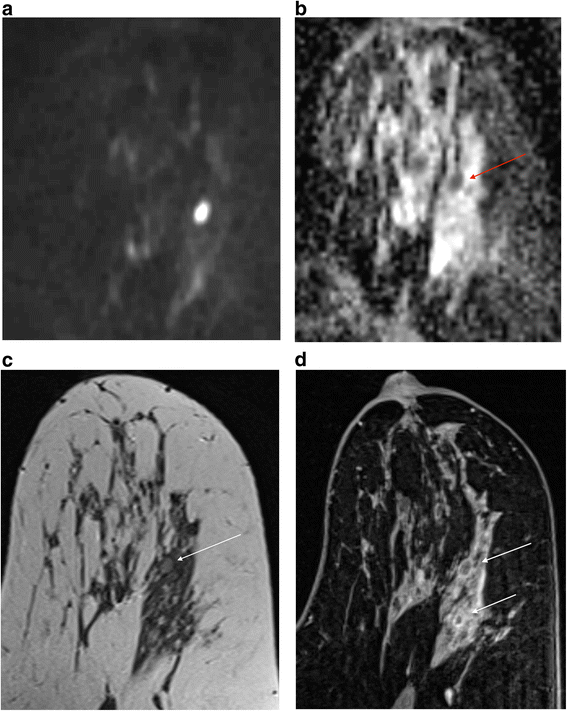
If there is a T2 correlate I routinely look at the DWI and corresponding apparent diffusion coefficient (ADC) map. I utilize b values of 50 and 850. Whilst I take that DWI is not necessary for the estimation of breast MRI scans, there are occasions when it can exist very helpful, provided the serial is of acceptable quality. In the presence of a known cancer and florid BPE, working out exactly what is malignant and what is not can exist very hard and it is hither that the DWI can be helpful [26] (Fig. 4). However, if y'all are trying to evaluate a seven mm mass or non-mass enhancement in a fatty chest, and the DWI slices are 4 mm thick with a ii mm gap and poor fatty suppression, it is probably non going to yield any useful data. It may be enough merely to 'eyeball' the ADC map to establish whether there is restriction of diffusion, but I generally copy and paste a ROI from the loftier b value image to the ADC map. A catch to exist aware of is that certain high course tumours, particularly triple negative basal phenotype cancers, commonly have areas of necrosis and may therefore have loftier whole-lesion ADC values [27]. Apply of a small ROI may be more discriminatory (Fig. 5). On the other hand, proteinaceous cysts may exhibit increased betoken at high b values, a low ADC and but intermediate T2 signal. In instances similar this a quick glance at the DCE series enables the correct diagnosis (Fig. 6). Information technology is also of import to be aware of the presence of any marker clips or staples, where susceptibility artefacts preclude useful ADC measurements.
Multifocal carcinoma in a patient with florid BPE (same patient as in Boosted file 1: Effigy S4b). First (a) and second (b) post-contrast subtractions showing diminished tumour to background contrast in the second acquisition. c Delayed high resolution fat suppressed T1W epitome showing tumour at 12 o'clock (solid arrow) and florid BPE particularly at four o'clock (dashed arrow). Notation similar signal intensities in the two areas. Centric T2W (d) and corresponding ADC map (east) show subtle T2 hyperintense tumour and obvious restriction of improvidence in the mass. Notation similarity of distribution of restricted diffusion to enhancing neoplasm in (a). Extent of neoplasm for treatment planning is well depicted in the sagittal reconstruction of the get-go post-contrast subtraction (f)
ADC measurement in a grade 3 triple negative breast cancer with some central necrosis. a b850 image (b) whole tumour ADC (c) 'hot spot' or ADCmin which is substantially lower
Cystic benign change. Axial b850 image (a), corresponding ADC map (b), T2 weighted image (c) and post-contrast T1 weighted epitome demonstrating restriction of diffusion in a proteinaceous cyst (d). There is an ovoid lesion with high b850 signal (a) and restricted diffusion (arrowed) (b). There is intermediate signal within it on T2W imaging (c) but the high resolution post-contrast sequence shows a small corporeality of enhancement around a cyst, with other cysts elsewhere (d)
The side by side step is kinetic and morphological analysis of any enhancement using the BI-RADS lexicon. In the latest edition, published in 2013 [28], the descriptors have been simplified and aligned with those in the mammography and ultrasound sections. It is possible to download a free pdf from the ACR BI-RADS website that summarises the MRI lexicon and I recommend having this to manus when reporting if y'all are not used to the descriptors. The main changes in the lexicon are summarised in Tabular array 2. Descriptors that were infrequently used (such every bit fundamental enhancement and enhancing internal septations) have been removed. Non-mass like enhancement becomes non-mass enhancement and the terms 'reticular/dendritic' and 'stippled' used to describe it have also been removed, equally they were used infrequently and stippled enhancement is recognised as a normal type of BPE. One add-on is the term 'amassed ring' to describe a form of non-mass enhancement often associated with DCIS, equally shown in Fig. 7.
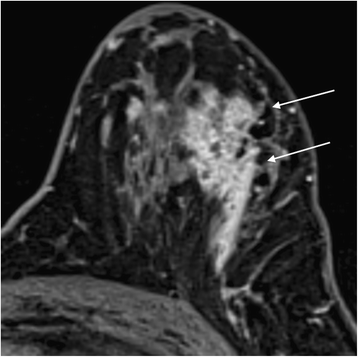
Clustered band enhancement in a patient with all-encompassing DCIS
When evaluating mass or non-mass enhancement I adopt to link the relevant series, equally shown in Fig. viii, so that multiparametric cess of whatsoever finding is facilitated. After I take made a morphological assessment using the BI-RADS descriptors, kinetic assessment follows. All of the major MRI manufacturers accept their own analysis packages which produce color overlays on the dynamic serial; generally speaking a lesion that is brilliant red is one that is enhancing rapidly, above a certain percentage threshold. These overlays are useful in drawing 1'south attention to areas of enhancement where time-intensity curves should exist drawn. I tend to move a pocket-sized region of interest around looking for the 'worst' curve; that is, rapid enhancement with washout (type iii curves). Often washout can be easily appreciated from the MIP serial, but of course if at that place is whatever move during the dynamic series (a frequent occurrence) it will non be possible to generate meaningful time-intensity curves unless there is very good motion correction (Fig. 3). It is for this reason that I ever evaluate the raw data likewise every bit the subtracted series. On the other paw, without the subtracted serial, high T1 point in the ducts could be wrongly interpreted equally segmental enhancement rather than the presence of proteinaceous fluid as occurs with duct ectasia.
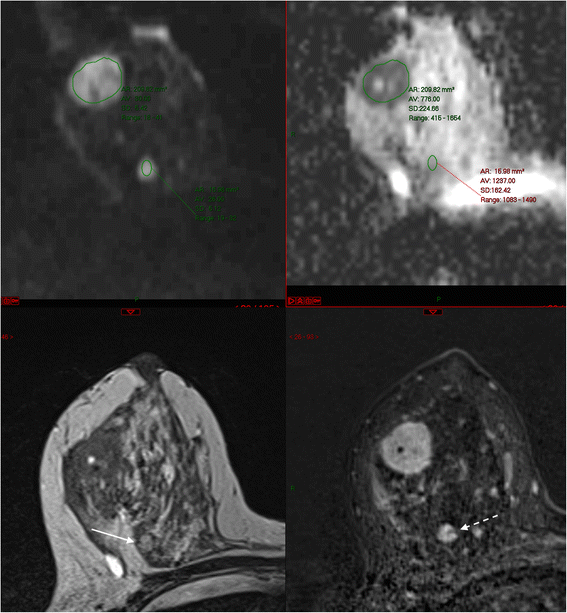
Multiparametric breast MRI. From top left to bottom right: DWI (b850), ADC map, T2W image and T1W postal service-contrast subtracted epitome. There is an obvious carcinoma in the upper outer quadrant of the right breast. An unexpected 2d rounded enhancing mass deep in the correct breast is slightly hyperintense on the T2 weighted image (pointer) and has high signal on the b850 image, simply there is no restriction of diffusion. Discover also a non-enhancing internal septation (dashed pointer). Biopsy-proven fibroadenoma
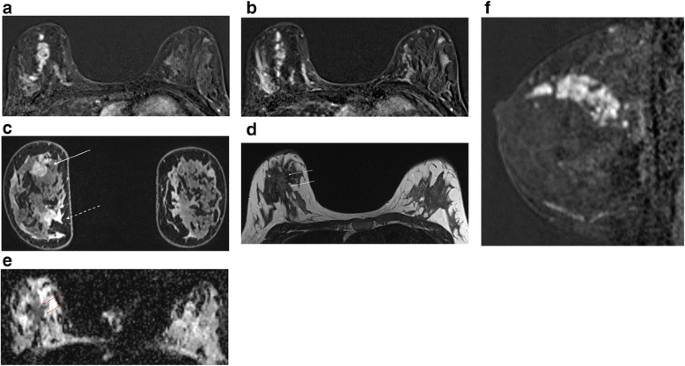
As a general principle it is most helpful to look at the early mail service-contrast subtractions to differentiate between malignancy and florid BPE (Fig. 4). During later acquisitions, there may be very piffling tumour-to-background contrast because of washout from the malignancy and persistent enhancement of BPE. Oftentimes, unsuspected foci of ipsilateral malignancy tend to have the same enhancement characteristics as the alphabetize lesion – though beware instances of ii different immunophenotypes within the same breast (Fig. 9).
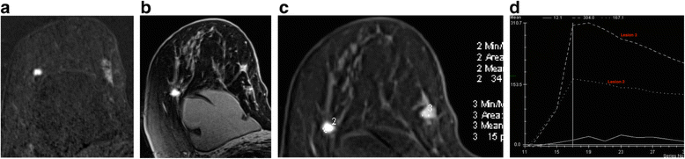
Patient with known form ane classical invasive lobular carcinoma in the upper inner quadrant of the right breast. MRI was indicated as the patient had breast implants and the breasts were difficult to assess with conventional imaging. Unexpected finding of a second carcinoma in the upper outer quadrant. a Postal service contrast subtracted image, (b) high resolution delayed post contrast paradigm, (c) regions of interest and (d) time-intensity curves. Note dissimilar morphology and kinetics of the two lesions; lesion 3 in the lateral breast was a hormone receptor positive class three invasive ductal carcinoma
Certain key measurements need to exist documented in the report. Not simply should the size of a lesion be given (in iii planes), but also the quadrant, clock face up position and distance from the nipple. Generation of 'route maps' in the advisable plane are helpful, non but for the surgeon but besides for the unfortunate radiologist or sonographer who may have to exercise a second-look targeted ultrasound. If there are small satellite lesions within a few mm of a known cancer I tend to include these in the overall dimensions, but if the MRI demonstrates farther lesions that were non expected, it is essential to document their location and their relationship with the index lesion; over again, reformatted route maps are very useful.
Earlier I terminate reviewing a study I take a mental checklist of review areas. The axilla of a cancer–bearing breast must be examined carefully and if in that location is obviously a heavy nodal burden the axillary apex and supraclavicular fossa should be reviewed. It is easy to miss enlarged lymph nodes in the internal mammary chain, an of import ascertainment as it may touch radiotherapy planning. Satisfaction of search is important; whereas it is difficult to overlook an enhancing lesion in the contralateral breast, it is very like shooting fish in a barrel to overlook pocket-sized bone metastases, liver lesions or lung nodules. All of these are commoner with a large chief neoplasm (T3 or 4) and N2 nodal disease and there is a specially high incidence with inflammatory breast cancer, a condition that is readily apparent with breast MRI.
Assigning a BI-RADS category
In MRI, BI-RADS 1 and 2 lesions have no probability of malignancy. Information technology is a question of preference whether to mention entities such every bit cysts, old scars or obvious fibroadenomas; if yous practice, y'all are bound to call these BI-RADS 2. I try e'er to minimise the number of category 3 (probably benign) lesions every bit these are a bit of an unknown quantity in chest MRI. Though in some retrospective serial, with variable follow-up and histopathological correlation, the rate of malignancy is low (effectually ii %) [29, 30], in other series it is around 4 % [31]. The presence of a T2 correlate, whatever restriction of diffusion and the size of the lesion can be helpful. There is evidence that the rate of BI-RADS 3 categorisation decreases with experience and with maturation of a screening programme; ideally the rate should be well under ten % and preferably nearer 3 %. BI-RADS 4 lesions have a probability of malignancy of betwixt 5–95 % and thus constitute a bit of a dumping footing; merely importantly, these lesions should not exist left lone. Information technology is here that correlation with not-dissimilarity MRI and conventional imaging can exist really helpful. For instance, if a modest mass has features of a lymph node, the presence of washout does not affair; this is a category 2 lesion. Fat necrosis can appear highly suspicious, with spiculate masses and washout kinetics, merely the diagnosis should be credible from evaluation not only of the not-dissimilarity images just as well conventional imaging.
In considering categorisation, morphology and kinetics should be considered together, simply morphology is the near important feature. Certain carcinomas may have type one curves (specially classical ILC), merely the morphology is usually highly suspicious. Kinetic analysis may not exist possible at all with linear non-mass enhancement as seen with DCIS, specially if in that location has been whatever movement. Conversely, some myxoid fibroadenomata may have washout curves; here the T2 correlate and DWI point is very useful. The morphological characteristic with highest PPV for malignancy is spiculation, followed by irregular shape or margin, and heterogeneous or rim enhancement [32–34]. Clumped nodular and clustered ring enhancement are the features of non-mass enhancement with the highest PPV for malignancy [31, 35]. On the other hand, circular or oval, smoothen non-enhancing masses or masses with non-enhancing internal septations are virtually never malignant. Finally, BI-RADS 5 lesions have a greater than 95 % chance of malignancy. In the U.s., a known biopsy proven carcinoma is category 6, though this category tends not to be used in the Uk. I can think of only a handful of occasions when I struggled to assign this category to a known invasive carcinoma; 2 were mucinous carcinomas, and the remainder were very small screen-detected ILC that barely enhanced at all. On the other manus, though some authors report an exceptionally loftier sensitivity of MRI for DCIS, especially loftier course [10], it is not uncommon to miss intermediate and low grade DCIS specially if the scans are poor quality.
It is important to remember that even in the presence of a known malignancy, multiple small-scale enhancing foci are nigh ever beneficial [31] and I endeavor not to overcall these. Otherwise there is the chance of overstaging, especially with invasive lobular carcinoma. Patients undergoing local staging will commonly take had image guided biopsy, which tin can results in peritumoural stranding, and (normally) mild enhancement – this should non be mistaken for the presence of an associated all-encompassing DCIS.
The study and management recommendations
As emphasised in the excellent introductory overview on how to read cancer imaging studies by Professor Hicks, probably the single most important factor in the issuing of a helpful report is a thorough understanding of the precise clinical question and of the factors that will influence the treatment plan. Above all, continue a sense of perspective – when a patient has a course 3, triple negative breast cancer that is shown on MRI to be locally advanced (T4) with obvious extensive nodal involvement, the presence of a pocket-size focus of non-mass enhancement that is indeterminate (BI-RADS MRI three) in the contralateral breast is to all intents and purposes irrelevant. The same is truthful of a similar focus in a dissimilar quadrant of the same breast since it is highly likely that the patient will ultimately crave mastectomy. On the other mitt, the decision to go for mastectomy should not be made on the basis of a second lesion without histological confirmation.
By and big, a BIRADS MRI 3 mass less than five mm or a focal non-mass enhancement nether x mm does not demand further evaluation [36]. Thus, recommending a 2nd look ultrasound or even MRI-guided biopsy may not be necessary, though in the Us this would more often than not mandate short interval (6 month) follow-upward. It helps to think about what you volition practice if yous cannot find the lesion on ultrasound; is there sufficient concern that MRI guided biopsy would then exist considered? If and so, it should probably be a category 4 lesion.
For BI-RADS 3 lesions that are larger than v mm (masses) or ten mm (nme) I would generally perform 2nd look ultrasound if information technology volition influence management. Reassuringly, the incidence of malignancy in lesions without a second await ultrasound correlate is relatively depression (though variable) [37, 38], merely attention to scan technique is critical. For BI-RADS 4 lesions, farther evaluation is always indicated. With 2nd look ultrasound, careful attention must be paid to altered spatial relationships. The sonographic correlates of the MRI lesion are frequently very subtle [39] and if anything is seen that might correspond to the lesion, it should exist biopsied and a marker clip inserted. I have found two techniques to exist helpful in this regard; firstly, the use of shear wave elastography to help identify subtle lesions and secondly, the use of ultrasound guided vacuum assisted biopsy. This is particularly helpful in cases of segmental nme, where the location of the abnormality is known. This volition ofttimes result in definitive histology. Failing that, MRI guided biopsy is necessary and this should be done in a timely fashion and so that there are no delays in the patient pathway.
For cases of known cancer staging, I give a T stage where possible. However, it is worth remembering that surgical management of a cancer depends non only on the absolute size of the lesion in relation to the size of the breast, just also on the site of the abnormality. A iii cm lesion can often be treated by wide local excision if it is situated in the upper outer quadrant of a big breast; but this volition not be the case if the lesion lies in the upper inner quadrant. Similarly, the orientation of the malignancy has a meaning impact on the treatment options. A lesion that is oriented radially towards the nipple tin often be resected even if information technology is over five cm in length (Fig. 10); whereas if the maximal diameter is in the coronal airplane, chest conservation will rarely be possible even with oncoplastic techniques. Finally, if the patient apparently has more than four lymph nodes that are involved by metastatic disease, I will recommend whole body staging if this has not already been carried out.
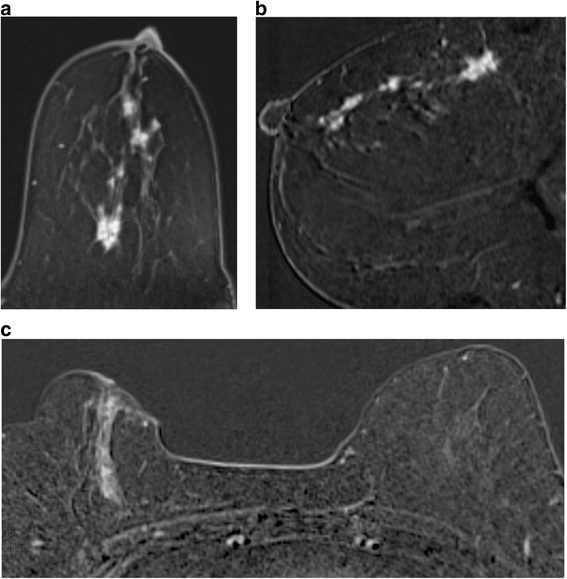
Two different patients with DCIS. a, b There is segmental clumped nodular enhancement over at least vi cm at 12 o'clock, extending to the nipple. c In that location is segmental linear non mass enhancement at ii o'clock in the right upper inner quadrant over iv.5 cm. Breast conservation was possible in the first case simply non in the second
There are instances where I pursue indeterminate findings much more aggressively; namely in the example of women with BRCA mutations undergoing screening MRI, particularly if there is a known or suspected BRCA 1 mutation. BRCA 1 cancers tend to grow extremely rapidly and have a singled-out phenotype; they often announced beneficial, being rounded and relatively well defined [forty]. Washout kinetics need not be nowadays when they are small and they tin look remarkably like fibroadenomas. This is one instance in which I may pursue masses under 5 mm in size, every bit these tumours have a very fast doubling charge per unit. If no lesion is found on 2nd look ultrasound I would and then propose either very short interval follow-up or MR guided biopsy. The management approach I utilize is summarised in Table 3.
Conclusions
Breast MRI is a remarkably powerful tool but if we are to do no damage, the onus is on us to appreciate the limitations of the technique and to issue a clear and concise report that details the level of concern and the deportment, if whatsoever, that need to exist taken. I am a bully believer in brevity when information technology comes to reports; I want my reports to be read! Therefore I tend not to exhaustively list all the scan parameters and all the normal/benign findings. If resources permit double-reporting, this is highly desirable especially when a service is existence introduced. Indeed, in the UK information technology is a quality requirement for high risk family history scans, which are carried out under custodianship of the NHS breast screening programme. Failing that, adoption of a standardised approach to reporting, such as the one I have discussed above, minimises the likelihood of errors or omissions. In summary:
-
Have a thorough agreement of the strengths and weaknesses of chest MRI
-
Accept a good indication and cull your patients carefully
-
Ensure the patient is well prepared
-
Understand the precise clinical question and the findings that volition alter handling
-
Use recognised descriptors in your study
-
Depict precisely where lesions are in relation to landmarks such every bit the nipple; give a T phase
-
Cheque all nodal stations carefully including apical and internal mammary lymph nodes
-
Recollect satisfaction of search; check the other breast!
-
Bank check for extramammary findings (lungs, bone visualised liver)
-
Give a concise report with a final assessment score and a clear management recommendation
Abbreviations
ADC, apparent diffusion coefficient; BI-RADS, breast imaging and reporting information arrangement; BPE, background parenchymal enhancement; DCE-MRI, dynamic dissimilarity-enhanced magnetic resonance imaging; DCIS, ductal carcinoma in situ; DWI, diffusion weighted imaging; FFDM, full field digital mammography; HRT, hormone replacement therapy; ILC, invasive lobular carcinoma; MIP, maximum intensity projection; NHS, National Wellness Service; TSE, turbo spin-echo; US, ultrasound
References
-
Britton P, Warwick J, Wallis MG, O'Keeffe S, Taylor K, Sinnatamby R, et al. Measuring the accuracy of diagnostic imaging in symptomatic breast patients: team and individual functioning. Br J Radiol. 2012;85:415–22.
-
Pisano ED, Gatsonis C, Hendrick Due east, Yaffe 1000, et al. Diagnostic functioning of digital versus picture mammography for breast cancer screening. N Engl J Med. 2005;353:1773–83.
-
Berg WA, Bandos AI, Mendelson EB, Lehrer D, Jong RA, Pisano ED. Ultrasound as the Primary Screening Examination for Chest Cancer: Analysis From ACRIN 6666. J Natl Cancer Inst. 2016;108(four):1–8.
-
Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of almanac screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404.
-
Mariscotti G, Houssami N, Durando M, Bergamasco L, Campanino PP, Ruggieri C, et al. Accurateness of mammography, digital breast tomosynthesis, ultrasound and MR imaging in preoperative cess of breast cancer. Anticancer Res. 2014;34(3):1219–25.
-
Luparia A, Mariscotti G, Durando M, Ciatto Southward, Bosco D, Campanino PP, et al. Accuracy of tumour size assessment in the preoperative staging of breast cancer: comparison of digital mammography, tomosynthesis, ultrasound and MRI. Radiol Med. 2013;118(7):1119–36.
-
Gruber 4, Rueckert M, Kagan KO, Staebler A, Siegmann KC, Hartkopf A, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging equally compared to histological tumour size in master breast cancer. BMC Cancer. 2013;thirteen:328.
-
Houssami N, Ciatto Southward, Macaskill P, Lord SJ, Warren RM, Dixon JM, et al. Accuracy and surgical touch on of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26(19):3248–58.
-
Brennan ME, Houssami N, Lord S, Macaskill P, Irwig L, Dixon JM, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27(33):5640–ix.
-
Kuhl CK, Schrading Southward, Bieling HB, Wardelmann East, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485–92.
-
Leach MO, Boggis CRM, Dixon AK, Easton DF, Eeles RA, Evans DGR, et al. Screening with magnetic resonance imaging and mammography of a United kingdom population at high familial take chances of chest cancer: a prospective multicentre accomplice study (MARIBS). Lancet. 2005;365(9473):1769–78.
-
Rijnsburger AJ, Obdeijn I-M, Kaas R, Tilanus-Linthorst MMA, Boetes C, Loo CE, et al. BRCA1-associated chest cancers nowadays differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC screening study. J Clin Oncol. 2010;28(36):5265–73.
-
Sardanelli F, Podo F, Santoro F, Manoukian S, Bergonzi S, Trecate Chiliad, et al. Multicenter surveillance of women at high genetic breast cancer chance using mammography, ultrasonography, and dissimilarity-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 written report): final results. Invest Radiol. 2011;46(ii):94–105.
-
Riedl CC, Luft N, Bernhart C, Weber M, Bernathova Yard, Tea M-KM, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, historic period, and chest density. J Clin Oncol. 2015;33(10):1128–35.
-
Houssami N, Turner R, Morrow Thousand. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257(2):249–55.
-
Ko ES, Han B-K, Kim RB, Ko EY, Shin JH, Nam SY, et al. Analysis of the effect of breast magnetic resonance imaging on the effect in women undergoing chest conservation surgery with radiation therapy. J Surg Oncol. 2013;107(eight):815–21.
-
Vos EL, Voogd AC, Verhoef C, Siesling S, Obdeijn IM, Koppert LB. Benefits of preoperative MRI in chest cancer surgery studied in a big population-based cancer registry. Br J Surg. 2015;102(13):1649–57.
-
Houssami Northward, Turner R, Macaskill P, Turnbull LW, McCready DR, Tuttle TM, et al. An individual person data meta-assay of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32(5):392–401.
-
Warner E, Hill K, Causer P, Plewes D, Jong R, Yaffe Thou, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–9.
-
Møller P, Stormorken A, Jonsrud C, Holmen MM, Hagen AI, Clark North, et al. Survival of patients with BRCA1-associated chest cancer diagnosed in an MRI-based surveillance plan. Breast Cancer Res Treat. 2013;139(i):155–61.
-
Isle of mann RM. The effectiveness of MR imaging in the cess of invasive lobular carcinoma of the breast. Magn Reson Imaging Clin Due north Am. 2010;18(two):259–76.
-
Langlands F, White J, Kearins O, Cheung S, Burns R, Horgan Chiliad, et al. Contralateral breast cancer: incidence according to ductal or lobular phenotype of the primary. Clin Radiol. 2016;71(2):159–63.
-
Trimboli RM, Verardi N, Cartia F, Carbonaro L, Sardanelli F. Chest cancer detection using double reading of unenhanced MRI including T1-weighted, T2-weighted STIR, and diffusion-weighted imaging: a proof of concept written report. Am J Roentgenol. 2014;203(3):674–81.
-
Hambly NM, Liberman L, Dershaw DD, Brennan Southward, Morris E. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-upward. AJR Am J Roentgenol. 2011;196(1):218–24.
-
DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on chest MRI: impact on diagnostic performance. AJR Am J Roentgenol. 2012;198(iv):W373–lxxx.
-
Pinker K, Bickel H, Helbich TH, Gruber S, Dubsky P, Pluschnig U, et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the "Chest Imaging Reporting and Data System" for multiparametric 3-T imaging of breast lesions. Eur Radiol. 2013;23(7):1791–802.
-
Youk JH, Son EJ, Chung J, Kim J-A, Kim Due east-G. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol. 2012;22(8):1724–34.
-
Morris EA, Comstock CE, Lee CH, et al. Magnetic resonance imaging. In: ACR BI-RADS® atlas, breast imaging reporting and information system. 5th ed. Reston: American College of Radiology; 2013.
-
Lourenco AP, Chung MTM, Mainiero MB. Probably benign breast MRI lesions: frequency, lesion type, and charge per unit of malignancy. J Magn Reson Imaging. 2014;39(4):789–94.
-
Spick C, Szolar DHM, Baltzer PA, Tillich Chiliad, Reittner P, Preidler KW, et al. Rate of malignancy in MRI-detected probably benign (BI-RADS iii) lesions. AJR Am J Roentgenol. 2014;202(iii):684–ix.
-
Grimm LJ, Anderson AL, Bakery JA, Johnson KS, Walsh R, Yoon SC, et al. Frequency of malignancy and imaging characteristics of probably benign lesions seen at breast MRI. AJR Am J Roentgenol. 2015;205(2):442–7.
-
Agrawal G, Su One thousand, Nalcioglu O, Feig Southward, Chen J. NIH public access. Cancer. 2010;115(164):1363–80.
-
Mahoney MC, Gatsonis C, Hanna Fifty, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology. 2012;264(ane):51–viii.
-
Pinker-Domenig Chiliad, Bogner W, Gruber S, Bickel H, Duffy S, Schernthaner M, et al. High resolution MRI of the breast at iii T: which BI-RADS® descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol. 2012;22(2):322–30.
-
Tozaki M, Igarashi T, Fukuda K. Chest MRI using the VIBE sequence: amassed band enhancement in the differential diagnosis of lesions showing non-masslike enhancement. AJR Am J Roentgenol. 2006;187(two):313–21.
-
Dall BJG, Vinnicombe S, Gilbert FJ. Reporting and management of breast lesions detected using MRI. Clin Radiol. 2011;66(12):1120–8.
-
Abe H, Schmidt RA, Shah RN, Shimauchi A, Kulkarni K, Sennett CA, et al. MR-directed ("Second-Look") ultrasound exam for breast lesions detected initially on MRI: MR and sonographic findings. AJR Am J Roentgenol. 2010;194(two):370–seven.
-
Spick C, Baltzer PAT. Diagnostic utility of second-await U.s. for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology. 2014;273(2):401–9.
-
Nam SJ, Kim E-K, Kim MJ, Moon HJ, Yoon JH. Significance of incidentally detected subcentimeter enhancing lesions on preoperative breast MRI: function of 2d-look ultrasound in lesion detection and management. AJR Am J Roentgenol. 2015;204(iii):W357–62.
-
Gilbert FJ, Warren RML, Kwan-Lim Chiliad, Thompson DJ, Eeles RA, Evans DG, et al. Cancers in BRCA1 and BRCA2 carriers and in women at high risk for breast cancer: MR imaging and mammographic features. Radiology. 2009;252(ii):358–68.
Acknowledgements
Cheers to Dr Shelley Waugh, PhD, for help in prototype assay.
Funding
None.
Availability of data and materials
Not applicable.
Author contribution
SJV responsible for the formulation, drafting and revision of the manuscript.
Competing interests
The author declares there are no competing interests.
Consent for publication
All patients routinely give consent for employ of their anonymised images for teaching and research purposes at my institution.
Ethics approval and consent to participate
Non applicable.
Author information
Affiliations
Respective author
Additional file
Boosted file 1: Effigy S4.
Background parenchymal enhancement. a Axial post contrast subtracted paradigm; minimal BPE in the right breast and a big enhancing cancer in the left upper inner quadrant (same patient as in Fig. 2). b Axial MIP serial post intravenous dissimilarity in a patient with known correct breast cancer, for staging MRI. Severe BPE. c MIP image in a patient who has previously had radiotherapy to the right breast. Notation absenteeism of BPE in the right chest compared to the left. (ZIP 4210 kb)
Rights and permissions
Open up Admission This article is distributed under the terms of the Artistic Commons Attribution four.0 International License (http://creativecommons.org/licenses/past/four.0/), which permits unrestricted use, distribution, and reproduction in whatsoever medium, provided you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were fabricated. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/nada/1.0/) applies to the data fabricated bachelor in this article, unless otherwise stated.
Reprints and Permissions
About this commodity
Cite this commodity
Vinnicombe, S. How I report breast magnetic resonance imaging studies for breast cancer staging and screening. Cancer Imaging 16, 17 (2016). https://doi.org/10.1186/s40644-016-0078-0
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1186/s40644-016-0078-0
Keywords
- Magnetic resonance imaging
- Breast cancer
- Staging
- BI-RADS
- Screening
Source: https://cancerimagingjournal.biomedcentral.com/articles/10.1186/s40644-016-0078-0
Belum ada Komentar untuk "How Do I Qualify to Read Breast Mri"
Posting Komentar